Nebraska Children’s Hospital Researchers Publish Study Demonstrating Successful Use of Ascents® Clinical Aromatherapy to Decrease Pain and Nausea, Improve Mood
Dr. Meaghann Weaver of Children’s Hospital & Medical Center (Omaha, Nebraska) recently published the results of her palliative care study utilizing Aeroscena®'s Ascents®-brand clinical aromatherapy inhalers in Cambridge University Press’ journal Palliative and Supportive Care. Researchers found that the three Ascents aromatherapy formulas used in the study represented an effective supportive care intervention for pediatric patients experiencing pain, nausea, and anxiety.
CLEVELAND (PRWEB) August 27, 2019
New research[1] carried out by Dr. Meaghann Weaver at Children’s Hospital & Medical Center (Omaha, Neb.) has demonstrated that Aeroscena's Ascents-brand clinical aromatherapy formulas represent an effective supportive care intervention for pediatric patients experiencing pain, nausea and anxiety.
Entitled, "Aromatherapy improves nausea, pain, and mood for patients receiving pediatric palliative care symptom-based consults: A pilot design trial," and published in Cambridge University Press’s academic journal "Palliative and Supportive Care," the study utilized three distinct Ascents clinical aromatherapy formulas: Calm No. 34, Nausea Relief No. 44, and Focus No. 04. Personal inhaler sachets of each formula were tested as supportive care interventions to decrease pediatric patients’ experience of stress/anxiety, nausea and emesis, and pain, respectively.
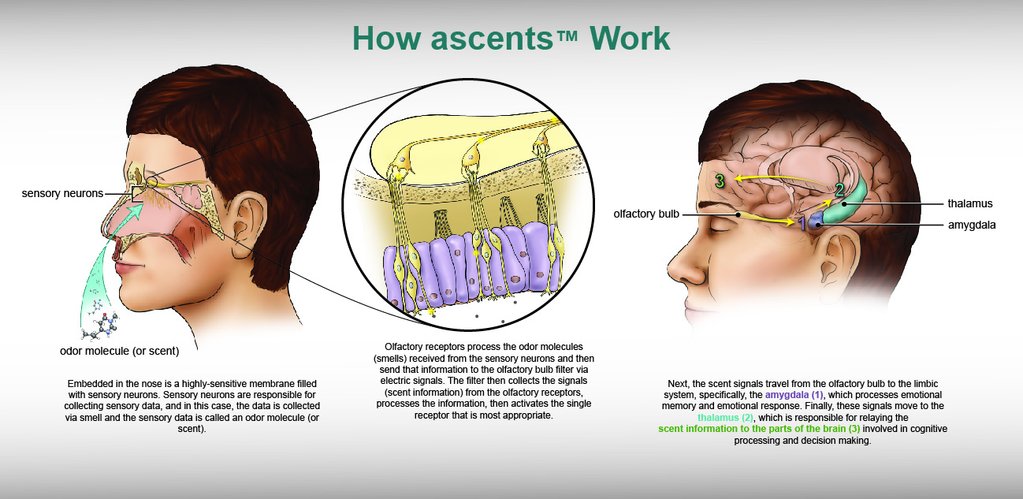
Ascents Inhalers: A Unique Aromatherapy Delivery System Designed for the Needs of Clinical Environments
In selecting the mode of aromatherapy delivery, the pediatric palliative care team engaged in an extensive review of aromatherapy product formats used in various health care settings. While many previous studies utilized vials of liquid essential oils, concern for contamination, dermatologic impact, accidental ingestion, and spills led Dr. Weaver’s team to Ascents inhalers. Ascents’ sachet format of aromatherapy was selected because of its individual, localized, and non-topical use, as well as overall high safety profile and minimal likelihood of eliciting an allergic reaction due to the oils’ distillation processes. Additionally, the sachets adhere to rigorous manufacturing purity, safety and quality standards; their unique design enables consistent dosing and preservation of essential oils; and they are easy to use, even for those patients with limited physical ability, strength, or range of motion.
Once chosen, Ascents inhalers were presented to the hospital infection prevention team and value analysis team for their review and approval prior to the study’s start.
The Study: Objective, Results and Conclusions
The objective of Dr. Weaver’s study was to investigate the role of aromatherapy in supportive symptom management for pediatric patients receiving palliative care. The research aimed to measure the impact of aromatherapy using validated child-reported nausea, pain, and mood scales 5 minutes and 60 minutes after aromatherapy exposure.
Symptom burden was sequentially assessed at 5 and 60 minutes using the Baxter Retching Faces (BARF) scale for nausea, the Wong-Baker FACES scale for pain, and the Children’s Anxiety and Pain Scale (CAPS) for anxious mood. At 5 minutes, significant improvements were reported for each of the three symptom sets, and remained improved at 60 minutes post-intervention.
Based on the study’s strong, positive results, Dr. Weaver concluded that aromatherapy represents a feasible, implementable supportive care intervention for pediatric patients receiving palliative care consults for symptom burden including nausea, pain and anxiety.
"We could not be more pleased with the outcomes of Dr. Weaver's study," said Mark Kohoot, Founder and CEO of Aeroscena. "Knowing that our products are effective in providing comfort care -- especially to children -- gives us great pride, and we will continue to utilize our unparalleled research platform to further the scientific understanding of phyto-pharmaceuticals for use in clinical environments."
[1]Weaver MS, Robinson J, Wichman C (2019). Aromatherapy improves nausea, pain, and mood for patients receiving pediatric palliative care symptom-based consults: A pilot design trial. Palliative and Supportive Care, 1–6.
About Aeroscena®
Established in 2010, Aeroscena is the corporate research and development organization behind Ascents®, the leading brand of therapeutic phyto-inhalants™. Aeroscena has developed the first scientifically-recognized platform for essential oils R&D. Ascents-branded clinical aromatherapy formulas are "recommended by doctors, used by hospitals®". Aeroscena is located in the Cleveland Clinic Global Cardiovascular Innovations Center in Cleveland, OH. For more information about Ascents, visit https://www.shopascents.com. For more information about Aeroscena®, visit https://www.aeroscena.com.
About Children’s Hospital and Medical Center
Children’s Hospital & Medical Center is the only full-service, pediatric health care center in Nebraska, providing expertise in more than 50 pediatric specialty services to children across a five-state region and beyond. Children’s is home to Nebraska’s only Level IV regional Newborn Intensive Care Unit and the state’s only Level II Pediatric Trauma Center. A regional heart center, it also offers expertise in pediatric heart transplantation. Children’s is recognized as a 2019-20 Best Children’s Hospital by U.S. News & World Report in five pediatric specialties: Cardiology and Heart Surgery, Pulmonology, Gastroenterology & GI Surgery, Orthopedics and Diabetes & Endocrine Disorders. Visit us online at ChildrensOmaha.org.